Justin Stebbing, professor of Biomedical Sciences at Anglia Ruskin University and editor-in-chief of Oncogene, explains why the vaccines could become a new therapeutic modality.
The success of vaccines against human papillomavirus (HPV) is staggering, practically eradicating cases of cervical cancer in the Western world going forward. It has prevented an infectious disease in that case, HPV, which causes cervical cancer.
But this year is probably also going to be a big year for cancer vaccines, which use a different type of technology to treat cancer patients. Might 2025 be the year that we see definitive evidence of cancer vaccines that herald a new era of personalised therapy?
There have, after all, been so many failures in the cancer vaccine space. But one of the differences is that these modern versions work via messenger RNA (mRNA) molecules which encode small amounts of protein leading to an immune response.
It’s this sort of technology that led Katalin Karikó and Drew Weissman to win the 2023 Nobel Prize in Medicine for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against Covid-19.
Personalised cancer vaccines provide highly targeted treatment based on a biopsy of the patient’s own cancer, but their complex manufacturing process makes them expensive and tricky to make, especially in a short timeline to get it back to the patient, those that need it most.
The data we’ve seen so far seems incredibly promising, but with so many past failures, nothing should be taken for granted.
How mRNA cancer vaccines work?
mRNA vaccines are a type of nucleic acid-based vaccine that contains mRNA which can encode antigens – usually tumour-associated antigens (TAAs) or tumour neoantigens – that can be delivered to the immune system via different delivery mediums.
Tumour samples are first taken from an individual which can then be sequenced to identify tumour-associated antigens (TAAs) or tumour neoantigens. Once the most immunogenic antigens are selected, these can be designed into mRNA sequences that can encode into those specific antigens selected. The mRNA sequence is usually encapsulated by lipid nanoparticles which are then injected into the individual; it is then translated to create the TAAs/tumour neoantigens that stimulate an immune response. Unlike preventative vaccines which rely on B cells to produce antibodies via a humoral response, a potent T-cell response is needed in order to attack the tumour in therapeutic cancer vaccines. This has analogies with the mRNA vaccines used for Covid-19 during the pandemic.
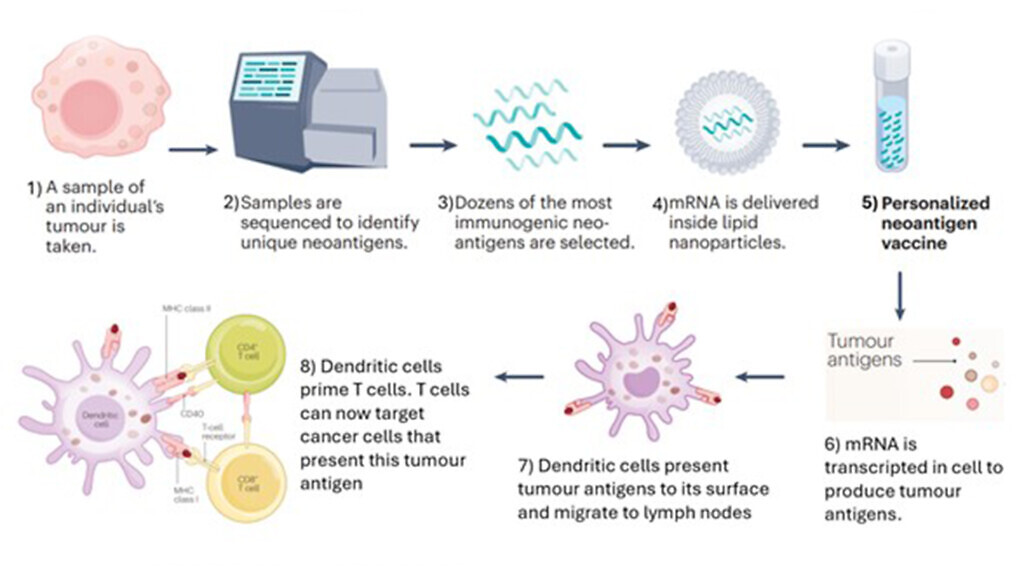
What are mRNA cancer vaccines useful for?
Physicians have long attempted to harness the power of the immune system to treat cancer. As described above, we now have technology that enables us to vaccinate patients with mRNA that can be encoded to TAAs/ tumour neoantigens. This also creates the advantage of the production of personalised vaccinations, as we can target specific TAAs/tumour neoantigens via tumour exome analysis of individuals’ tumour samples.
Here it is useful to note that mRNA vaccines can provide an advantage of being able to allow multiple antigens to be administered in one immunisation, meaning it can be possible to target multiple antigens at once depending on an individual’s unique tumour profile. Another use includes cellular therapies which use transfection of patient-derived cells, for example, dendritic cells in vitro
These are antigen-presenting cells (APCs) and can prime T cells to recognise a particular antigen which stimulates an adaptive immune response. Instead of exposing dendritic cells to proteins, they can be exposed to mRNA encoding TAAs/neoantigens which allows the dendritic cells to produce the tumour antigen themselves and present these antigens on its surface. This in turn primes T cells so that the immune system can recognise the antigen and attack cancer cells that express these antigens.
An advantage of this approach is that it reduces the risk of autoimmunity as we are avoiding growing patient-specific tumours as patient-specific tumours contain patients’ normal self-antigens that can be recognised by the immune system. A limitation to using mRNA encoding into TAAs is that we don’t know the TAAs for many cancers, which creates problems as we need to identify the suitable TAAs needed when designing mRNA vaccines.
Chimeric antigen receptor (CAR) T cell therapy uses patient-derived T cells which are genetically engineered to express receptors that target specific TAAs and therefore can attack specific tumours. mRNA here, can be used to help engineer these T cells through methods such as mRNA electroporation which has been undertaken in a Phase I trial with two patients with solid malignancies (one with advanced mesothelioma and the other having metastatic pancreatic cancer), by way of one example.
In one of the most exciting use cases to date, mRNA cancer vaccines have shown dramatic improvements in outcomes when combined with immune checkpoint blockade in patients with Stage III melanoma.
For example, a randomised Phase IIb trial of a personalised mRNA neoantigen vaccine with an immune checkpoint inhibitor (pembrolizumab) for high-risk melanoma showed improved recurrence-free survival compared to pembrolizumab alone. In the overall intention-to-treat (ITT) population, adjuvant treatment with mRNA-4157 (V940) in combination with pembrolizumab demonstrated a statistically significant and clinically meaningful improvement in distant metastasis-free survival compared with pembrolizumab routine care alone and reduced the risk of developing distant metastasis or death by 65%.
A Phase I trial of a personalised adjuvant mRNA neoantigen vaccine for pancreatic cancer showed those who responded to the vaccine in terms of a T cell response had a longer median recurrence-free survival compared to non-responders to the vaccine.

How can mRNA cancer vaccines impact care?
Going forwards, this requires an analysis of tumour cell samples from individuals and subsequently the sequence/design mRNA cancer vaccines, and giving this to the patient as a therapy. Again, having this personalisation may mean that we can more effectively treat cancer patients as we are targeting antigens unique to each patient’s specific tumour profile.
So far, they seem to be well tolerated. Because mRNA vaccines target specific TAAs/tumour neoantigens, it may have fewer side effects compared to current treatments such as chemotherapy or radiotherapy. This helps not only to improve the quality of life of cancer patients but also their quantity of life.

What stage has the technology reached in the UK?
The UK Government has partnered with BioNTech, a German company known for its work with Pfizer on mRNA vaccines against SARS-CoV-2 during the Covid-19 pandemic. This long-term partnership may allow access to precision cancer treatments for up to 10,000 patients in the UK by 2030.
One of the aims of this partnership is to establish the Cancer Vaccine Launch Pad whereby potentially eligible cancer patients are identified and a database is created for those who are suitable to participate in personalised vaccine trials. New facilities and staff are included in the plans to help support this project such as new laboratories in Cambridge.
NHS patients are currently being enrolled for a Phase II BioNTech mRNA cancer vaccine trial for high-risk resected colorectal cancer at University Hospitals Birmingham NHS Foundation Trust and other sites are expected to enrol more patients after 2026. As mentioned above, the Phase IIb study of a personalised mRNA neoantigen vaccine (mRNA-4157) plus pembrolizumab vs pembrolizumab monotherapy for resected high-risk melanoma showed patients with combination therapy had a 49% reduction in risk of recurrence or death after three years compared to the monotherapy standard treatment.
Following from the promising results of this trial, it has now expanded to a Phase III global clinical trial with an aim to recruit 1,100 people.
Depending on the success of the trial, it is possible we may see mRNA cancer vaccines being offered as a potential treatment option in the future – especially in the case of these patients who have high-risk resected melanoma.
Moderna will file with the US Food and Drug Administration (FDA) when it has initial results of its Phase III trial, aiming to decrease the recurrence rate after surgery. Large trials in addition to standard of care have commenced in common tumour types such as non-small cell lung cancer, and a key question in the future is whether mRNA cancer vaccines have monotherapy clinical activity, or require use in combination.
Either way, it is exciting times and my view is that mRNA vaccines will become part of standard of care in years to come, a completely new therapeutic modality.



