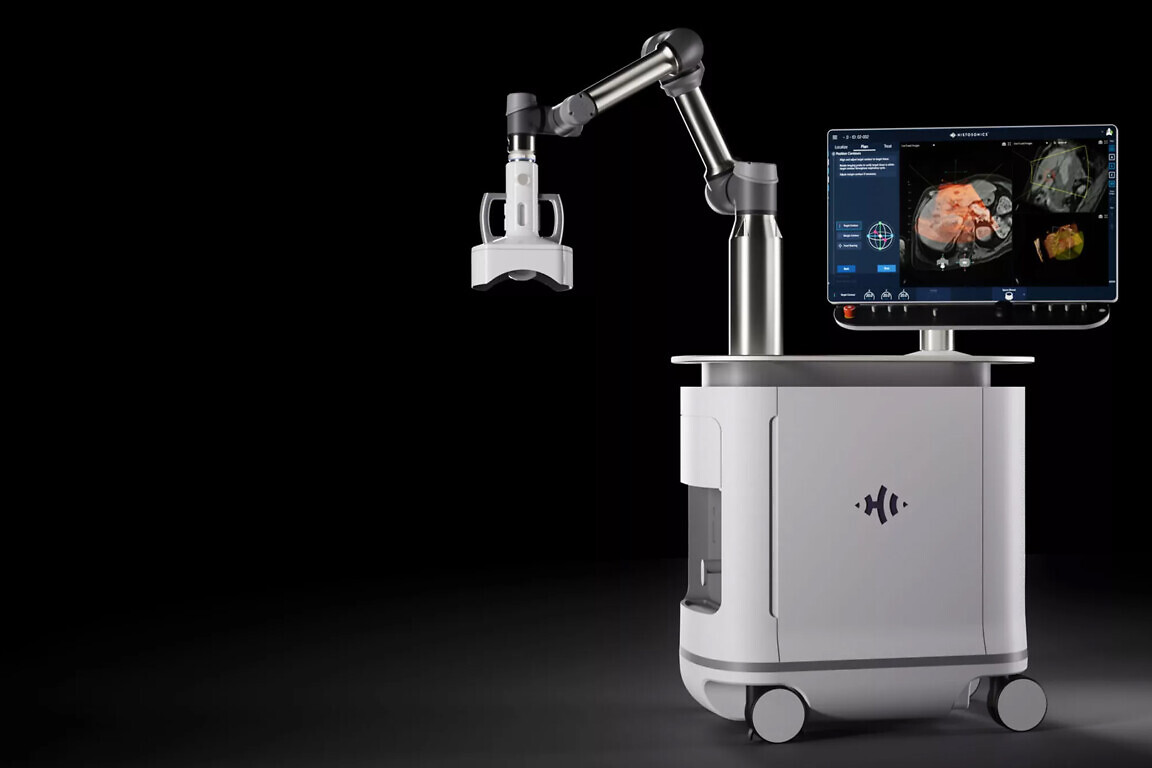
Developed by US-based company HistoSonics and launched at Addenbrooke’s Hospital in Cambridge in the summer, the ultrasound technology is delivered through a single session.
NHS patients will be the first in Europe to benefit from a new non-invasive liver cancer treatment which uses ultrasound technology to destroy tumours without surgery, scalpels or radiation, with minimal damage to surrounding organs.
Developed by US-based company HistoSonics, the treatment is being debuted at Addenbrooke’s Hospital in Cambridge, part of Cambridge University Hospitals NHS Foundation Trust, this summer. The technology was procured and installed thanks to a generous donation to the University of Cambridge from the Li Ka Shing Foundation, which has been a longstanding supporter of cancer research at the university.
Treatment is delivered through a single short session – potentially taking no longer than 30 minutes – with limited or no pain, a quick recovery and can be performed as a day case.
“By offering this non-invasive, more targeted treatment we can care for more people as outpatients and free up time for surgeons to treat more complex cases,” said Roland Sinker, chief executive of Cambridge University Hospitals
“The faster recovery times mean patients will be able to return to their normal lives more quickly, which will also reduce pressure on hospital beds, helping us ensure that patients are able to receive the right treatment at the right time,” he continued.
Faster recovery times
Patients stand to benefit from faster recovery times, potentially greater survival rates, fewer potentially dangerous complications and fewer hospital stays – helping to cut waits for others – all marking a new era in cancer treatment.
Ongoing research is exploring its potential to transform treatment for other hard-to-reach tumours, including kidney and pancreatic cancers, bringing hope to even more NHS patients in the future.
Health and social care secretary Wes Streeting granted authorisation for controlled early access to the device through an unmet clinical need authorisation. This is available through the UK’s Innovative Devices Access Pathway programme, a government-funded scheme to get health innovations to the market much more quickly.
“This is a strong example of smart, agile regulation in action. Working closely with partners through the Innovative Devices Access Pathway, we’ve shown we can get promising technologies to patients faster – without compromising safety,” said James Pound, interim executive director of innovation and compliance at the Medicines and Healthcare products Regulatory Agency.